Abstract
Background: Treatment options that became available over the past two decades, including proteasome inhibitors (PI) and immunomodulatory agents (IMIDs), have significantly improved survival outcomes of patients with multiple myeloma (MM). However, MM is still an incurable disease and patients ultimately progress and become refractory to standard treatments. The increased use of Lenalidomide regimens in earlier lines of treatment has led to a higher number of Lenalidomide (Len) refractory patients (Moreau P, Blood Cancer J 2019).
Description of characteristics and outcomes of patients who have become refractory to Len vs. those who have only been exposed but did not become refractory is limited especially with regards to data coming from different European healthcare systems.
Methods: Data from two MM registries, the Registry of Monoclonal Gammopathies from the Czech Republic (Brozova L, Klin Onkol. 2017) and the Oncology Information Service registry in Germany (Merz M, Cancers 2021), were analyzed in the Hematology Outcomes Network Europe (HONEUR). HONEUR is a federated data network with patient level data solely stored and governed at local sites with analysis executed centrally. MM patients exposed to both PI and Len, in 1-3 prior lines of therapy and with ECOG 0-1, treated between 2015 and 2021, were identified to compare outcomes of Len-exposed, non- refractory vs. Len-refractory patients.
Refractoriness to Len was defined as disease progression while on Len and/or initiation of subsequent line of therapy not containing Len within 60 days after stopping the previous Len containing line of treatment. Index dates were defined as the date of treatment initiation after meeting above criteria. Patients fulfilling the eligibility criteria at multiple times in their longitudinal follow-up were included as separate observations with line of therapy as unit of analysis.
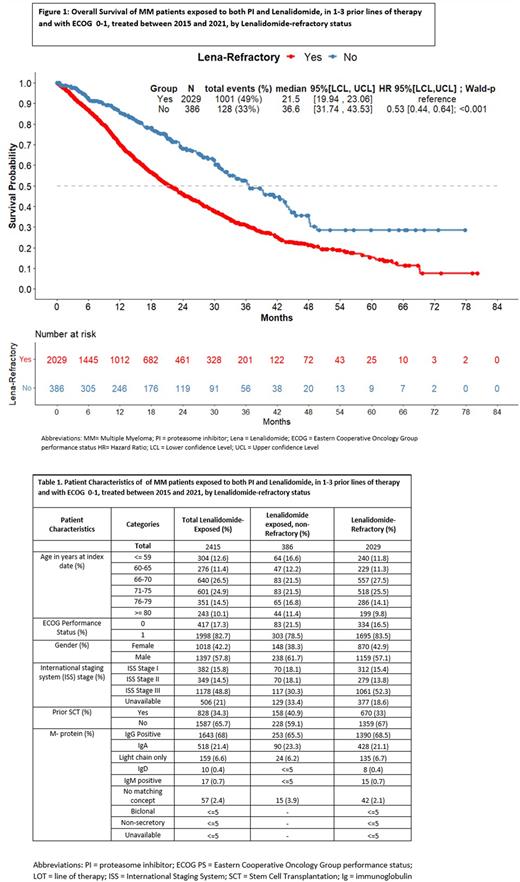
Results: A total of 2415 treatment lines were included in the analysis. Of these, 2029 (84.0 %) treatment lines were from Len-refractory patients. Median follow-up from index date was 22.0 vs 23.5 months for the Len-exposed non- refractory and Len-refractory patients, respectively. Baseline characteristics of the Len-exposed non-refractory and Len-refractory patients are presented in table 1. For Len-exposed non-refractory and Len-refractory patients, median OS was 36.6 [95% CI: 31.74; 43.53] vs. 21.5 [95% CI :19.94; 23.06] months, respectively, with a Hazard Ratio (HR) of 0.53 [95% CI: 0.44; 0.64] in favor of Len exposed non-refractory patients. When accounting for line of treatment, the adjusted hazard ratio was 0.59 [95% CI 0.49; 0.70] in favor of Len-exposed non- refractory patients.
Conclusions: An analysis of German and Czech registries in a federated data network shows that patients who have become refractory to Len have significantly poorer overall survival compared to Len-exposed non-refractory patients with MM. The Len-refractory population is a more difficult-to-treat patient population, with high unmet need for effective treatments that can prolong survival outcomes for these patients.
Disclosures
Hajek:Takeda: Consultancy, Honoraria; PharmaMar: Consultancy, Honoraria; Norvatis: Consultancy; BMS: Consultancy, Honoraria; AbbVie: Consultancy; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Sliwka:Janssen: Current Employment. Spicka:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaMar: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Minarik:TAKEDA: Consultancy, Honoraria; SANOFI: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; GSK: Consultancy, Honoraria; EUSA Pharma: Consultancy, Honoraria; CELGENE: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Perualila:Janssen: Current Employment. Diels:Janssen: Current Employment, Current equity holder in publicly-traded company. van Speybroeck:Janssen: Current Employment. Erler-Yates:Janssen: Current Employment. Mendes:Janssen: Current Employment. Strobel:Pfizer: Current equity holder in publicly-traded company. Merz:BMS Celgene: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal